Kapural L, Vrooman B, Sarwar S, et al. " A Randomized, Placebo-Controlled Trial of Transdiscal Radiofrequency, Biacuplasty for Treatment of Discogenic Lower Back Pain
In the past, the vast majority of these experimental minimally invasive procedures,which includes biacuplasty, were not sufficiently tested by sound scientific methodology; however, in 2012, Kapural et al. stunned the research and medical community by completing and publishing the results of a new procedure designed to treat chronic discogenic pain – that procedure was called Intradiscal Biacuplasty (IDB). In a nutshell, IDB is a treatment that uses a heating-up technique (like frying an egg) in order to destroy the pain-carrying nerve fibers in the back of the disc, which are thought to be chronically inflamed and responsible for the chronic pain of patients in whom suffer discogenic low back pain. It is similar to the dreaded IDET technique but uses two RF probes instead of one.
If you understand what discogenic pain and a randomized placebo-controlled trial are, you may jump down to the part where I start explaining how this experiment was conducted [here]. If not, keep reading.
In order to understand this review, we first need to know what the heck discogenic pain is. As the name implies, discogenic pain is a cause of chronic lower back pain (and sometimes referred leg pain) that occurs when the pain-carrying nerve endings that live in the back of the disc (posterolateral aspect mostly) become irritated and inflamed after being exposed to degenerated material (cytokines and interleukin-1) as well as nuclear cells (these are foreign and are attacked by the body's immune system), which come from the center of the disc – i.e., the nucleus pulposus. Normally this inflammatory material is confined to the center of the disc. However, with wear and tear, increasing age and bad genetics, rips and tears (annular tears) can occur within the tissue that corrals the nucleus pulposus in place (the inner annulus fibrosus) and let loose this potentially inflammatory material, and once it hits the outer one third of the posterolateral disc, in some but not all patients, a horrible pain-generating inflammation can began which becomes untreatable and chronic. [read more about discogenic pain here].
So now that you understand what discogenic pain is about, let's talk about this paper and what it means. The beauty of this study was in its design: a blinded randomized placebo-controlled trial, which is the king of all experimental design– nothing produces stronger scientific evidence. What does that mean in plain English? It means that the design of this study took out of play the "placebo effect." I'm sure everybody has heard of this, but in case some of you haven't, a placebo effect occurs when a patient's pain or disease is cured only because he or she believes that they have had a treatment that will cure it, when in fact they have not – they were told they had a treatment but in reality it was a fake treatment, like a sugar pill or in this case a fake IDB procedure. Removing the placebo effect is really the only way you can tell if the actual treatment is making the patient better on its own, and is very rarely allowed in treatment of lumbar spine conditions.Blinded Randomization is also an incredibly powerful tool and means that participating patients were randomly placed into either the placebo group or the treatment group and did not know (i.e. blinded) which group they were in.
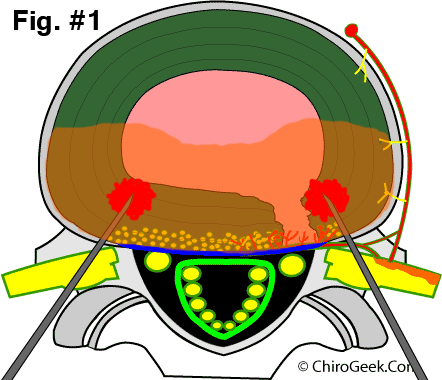
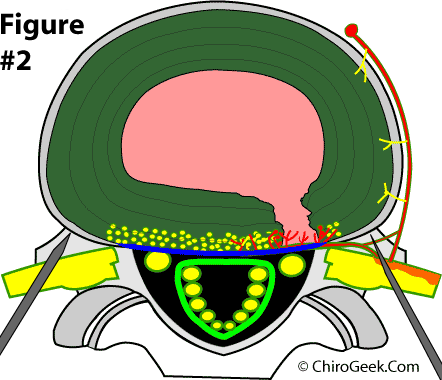
Figure #1. Overhead view of the L4 disc which has a symptomatic grade III (full thickness) annular tear. Two intradiscal introducers (gray needles) which contain the RF probes are on which is ablating (heating up) the annular tissue to the point that all nerve tissue is killed off and sealing of the annular tear may occur.
The light-orange zone represents the area that is heated by the tips of the RF probes. As you can see, a large portion of the disc is affected.
So what the heck is this IDB?
It is a new minimally invasive treatment for chronic discogenic low back pain which is done by inserting two special heat generating needles, under three dimensional fluoroscopic guidance, through the muscles of the low back and into the back of the disc (the part where all the pain-carrying nerve endings live) (Fig. #1) The RF probes are then turned on for 15 minutes in order to destroy, via thermal energy (like cooking an egg), the nerve endings, which is theorized to eliminate the source of discogenic pain and relieve the patient's pain.
Study Design: In order to qualify for the study, the potential patients had to have been diagnosed with chronic discogenic pain as confirmed by Provocative CT Discography. Other very strict entry criteria were employed and included no symptomatic disc herniations; no greater than two levels of involvement; symptoms of back pain greater than lower extremity pain; no secondary diagnoses of spondylolisthesis, stenosis, fibromyalgia, multilevel degenerative disc disease; and no involvement with litigation (i.e. no workers compensation or personal injury cases). Ultimately, 64 patients agreed to participate in the study and met the strict entry criteria. Next, all patients completed pre-procedure standard outcome assessment tools (the SF-36, Oswestry Disability Index [ODI], and a numeric rating scale [NRS] for back pain), which measure general quality of life, low back related disability, and pain, respectively. By blinded-randomization (luck of the draw), 32 patients were assigned to the real treatment group (treatment group) and the other 32 patients were assigned to the fake treatment group (placebo group). In both groups, three-dimensional fluoroscopy was used to guide two heat-generating devices (an RF probe inserted through a 17gauge TransDiscal introducer needle) obliquely through the muscles of the lower back toward the disc (one from the right and one from the left ). In the treatment group, the device was inserted into the substance of the posterolateral annulus fibrosus on each side (figure #1); whereas, in the placebo group, the device was left outside of the disc and the disc was not penetrated in any way(figure #2).
In both groups, the RF-generator (the device that creates the heat in the RF probes) was turned on so the patients had the same auditory experience during the procedure. In the treatment group, the RF generator actually delivered heat energy into the bilateral posterolateral aspects of the of the disc (most of the back of the disc) (figure #1); whereas, in the placebo group, no heat energy was generated at all. After 15 minutes of "cooking" the back of the disc (which theoretically destroyed the pain-generating nerve endings in the disc), the heat was turned off and this part of the experiment was over.
Six-Month Results: At the six month time-point (six months after the procedure was performed), over 80% of the patients in both groups were available to report early outcome of the procedure (84% in the treatment group and 87% in the placebo group) by completing the same outcome assessment tools. After their test taking was complete, the patient's were informed whether or not they actually had the biacuplasty. *Perhaps surprisingly (at least to me), analysis of the outcome assessment tool data revealed that the patients in the treatment group had statistically superior scores on the SF-36, ODI, and NRS as compared to the placebo group (p<0.04). In other words, the patients that had the IDB were doing better with regard to quality of life, level of back related disability, and magnitude of pain. However, not all of the results were supportive of the treatment group: with regard to opioid usage, although the treatment group patients did demonstrate an average 15.6 mg decrease, which was better than the 0 mg decrease in the placebo group, this difference was not statistically significant (which means that the difference could be simply to chance and not anything meaningful). In both groups there were no complications from the procedure, such as nerve root damage; severe flare-up or new pain; or infection of the disc (discitis).
Conclusions
The authors naturally concluded that IDB was an efficacious (effective and safe) treatment for chronic discogenic low back pain and was superior to that of placebo.
My 2 Cents
Hats-off to this investigative team who have reported early results of a new procedure for the treatment of the hard-to-treat discogenic pain syndrome of the low back via a randomized placebo-controlled trial, done at multiple clinics (n=2) with different physicians. Now this is the type of research that needs to be done on all invasive treatment modalities for the lumbar spine. Hopefully, others will follow their lead and test treatment interventions such as nucleoplasty, percutaneous discectomy, SED, etc, via the same most excellent methodology. It would be fantastic if we could get this type of study approved for at least a single level fusion for discogenic pain, but I doubt that will ever happen in this country.
The obvious limitations of the study include the incredibly short follow-up (six months is really nothing as most journals require a two-year minimal follow-up study), the relatively poor six-month patient participation (<88% in both groups) and the very strict entry criteria (they through out patients with multilevel degenerative disc disease, which knocked out the majority of the patients who suffer this condition). Nay-Sayers will focus on the fact that there was a nonstatistical difference between the groups with regard to decrease in opioid usage.
Although the placebo effect is now back in play (the patients were told which procedure they had), let's hope this team of researchers keep following the two cohorts in order to see what happens (although some of them have crossed over) farther on down the road. I still fear, based on the results of animal studies and the Carragee study, that by poking a hole in the disc (and in this case, two holes), that disc will be ultimately doomed to failure somewhere between the 5 and 10 year time-point (maybe even sooner since there were two holes made), which will ultimately necessitate fusion for those of whom suffer a relapse of intractable unrelenting lower back pain.
Would I try this treatment? If I were at the end of my rope, a candidate for interbody fusion, and rich (this treatment will not be covered by insurance), I probably would give it a try, as interbody fusion should be avoided as long as possible and at all reasonable costs.